Post By : 2025-05-21"
FDA may limit future Covid-19 shots to older people and those at risk of serious infection"
The US Food and Drug Administration is changing the way it approves Covid-19 vaccines for Americans, a move that may limit future shots to older Americans and people at higher risk of serious Covid-19 infection.
The agency is changing the type of evidence it will accept from vaccine manufacturers to approve updated Covid-19 shots, Dr. Vinay Prasad, the new director of the FDA’s Center for Biologics Evaluation and Research, and FDA Commissioner Dr. Marty Makary said in an editorial published Tuesday in the New England Journal of Medicine.
The change means the updated shots will probably be available this fall for adults 65 and older and those with underlying conditions that may put them at higher risk of a severe Covid-19 infection, but they may not be for everyone who was previously eligible. Nearly three-quarters of Americans 6 months and older have at least one of these underlying medical conditions, according to the US Centers for Disease Control and Prevention.
The change, which was already being studied by experts who advise the CDC on its vaccine recommendations, will more closely align the United States with guidelines in the UK, Canada and Australia.
But millions of healthy adults and kids stand to lose access to updated vaccines under the new criteria. Prasad and Makary say there’s not enough evidence that healthy children and adults get clinically meaningful benefit from regular Covid-19 shots, and they want to see more placebo-controlled trials, particularly in adults 50 to 64, before recommending the shots for other groups.
Does alcohol affect sleep? CNN reporter downed a martini and slept in a sleep lab to find out
3:09
Utah's ban on fluoride in public water goes into effect
3:03
The US Food and Drug Administration is changing the way it approves Covid-19 vaccines for Americans, a move that may limit future shots to older Americans and people at higher risk of serious Covid-19 infection.
The agency is changing the type of evidence it will accept from vaccine manufacturers to approve updated Covid-19 shots, Dr. Vinay Prasad, the new director of the FDA’s Center for Biologics Evaluation and Research, and FDA Commissioner Dr. Marty Makary said in an editorial published Tuesday in the New England Journal of Medicine.
The change means the updated shots will probably be available this fall for adults 65 and older and those with underlying conditions that may put them at higher risk of a severe Covid-19 infection, but they may not be for everyone who was previously eligible. Nearly three-quarters of Americans 6 months and older have at least one of these underlying medical conditions, according to the US Centers for Disease Control and Prevention.
The change, which was already being studied by experts who advise the CDC on its vaccine recommendations, will more closely align the United States with guidelines in the UK, Canada and Australia.
Can you recover from long Covid? Dr. Sanjay Gupta explains
2:02
But millions of healthy adults and kids stand to lose access to updated vaccines under the new criteria. Prasad and Makary say there’s not enough evidence that healthy children and adults get clinically meaningful benefit from regular Covid-19 shots, and they want to see more placebo-controlled trials, particularly in adults 50 to 64, before recommending the shots for other groups.
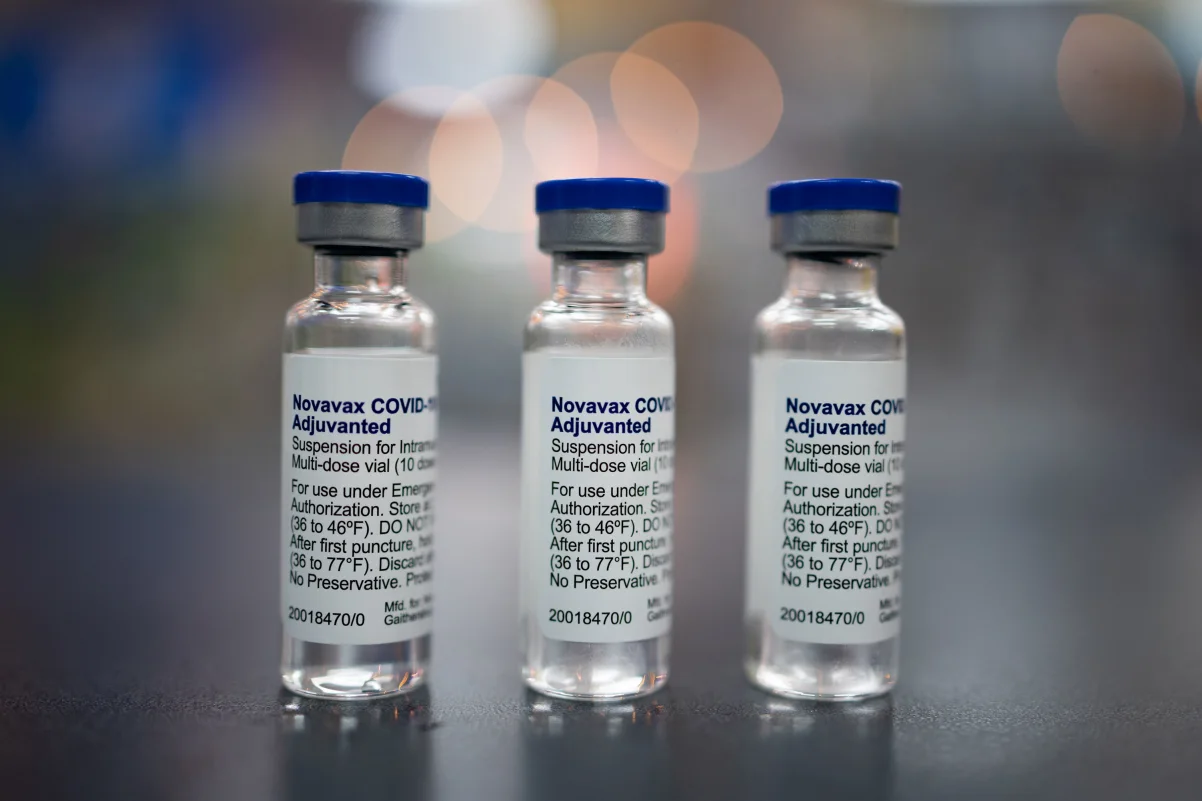
It’s not clear whether Covid-19 vaccine manufacturers, including Pfizer, Moderna and Novavax, will decide to conduct the randomized controlled trials the FDA is seeking for certain age groups. These kinds of studies are expensive and typically take months or even years.
Moderna said in a statement Tuesday, “We appreciate the FDA’s clear guidance and remain committed to working with the Agency to provide the data they need to ensure access for Americans.”
Pfizer and Novavax did not respond to requests for comment on the FDA’s new regulatory framework.
Just days before the FDA’s announcement, it approved the Novavax Covid-19 vaccine, which was six weeks past its planned approval deadline. The FDA restricted the use of the vaccine to people 65 and older and those 12 and up with underlying health conditions.
“Market research and US C.D.C. statistics indicate that older individuals and those with underlying conditions are the populations most likely to seek out COVID-19 vaccination seasonally,” Novavax President and CEO John Jacobs said at the time. “This significant milestone demonstrates our commitment to these populations and is a significant step towards availability of our protein-based vaccine option.”
‘We will consider the results’
In a discussion shared online Tuesday, Prasad answered some questions about the new requirements that were posed to him by Makary.
“This is a free country, and companies are, of course, free to conduct their own randomized studies in younger populations, in people without risk factors for severe Covid-19,” Prasad said. “They can run the research agenda they see fit, and we will consider the results of those studies.”
Prasad also sought to flesh out why the FDA settled on these age groups and risk factors for vaccine recommendations. For the 50-to-65 age group, “we are genuinely uncertain, globally, if those patients benefit,” he said, with some countries recommending Covid vaccination for people 65 and older and others as young as 40.
“Fifty to 65, we feel, is an area where there’s disagreement among our peer nations. It’s a place [where] we can bring data to this question,” he added.
Dr. Noel Brewer, a professor of public health and health behavior at the University of North Carolina at Chapel Hill, said he supports the change.
“The proposed policy moves the US in line with other countries. This global view of public health is a welcome development,” said Brewer, who sits on the CDC’s Advisory Committee on Immunization Practices and was part of the working group mulling the change to Covid vaccine recommendations.
"Copyright 2024 All Right Reserved By Urban Reports News